
|
Enquiries: contactATarmidaleDOTinfo
Thermodynamics
Ideal Gas Law
|
The Ideal Gas Law was dervied from three laws: Boyle's Law PV=k, stating that the Pressure of a gas multiplied by the Volume of that Gas equals some constant, or that Pressure is proportional to the inverse of Volume. This explains why pressure increases as the volume decreases, as there are more gas molecules within a smaller space. Charles's Law V/T=k, stating that the Volume of a gas divided by the Temperature of the Gas equals some constant, or that the Volume is proportional to the Temperature. This explains why a gas expands as it gets heated up. Gay-Lussac's Law P/T=k, stating that the Pressure of a gas divided by the Temperature of the Gas equals some constant, or that the Pressure is proportional to the Temperature. This explains why pressure inside a sealed container increases as it gets heated up. The Ideal Gas Law, PV=nRT, is a combination of these three laws. It states that the Pressure (P) multiplied by the Volume (V) is equal to the number of moles (n) multiplied by the Universal Gas Constant (R) multiplied by the temperature (T) [in Kelvin]. |
An important thing to keep in mind when making any of these calculations is to make sure you are using the correct units, otherwise your answer could go off the scales, especially since the units will often cancel out with the Universal Gas Constant. Here is an example problem and solution.
The
combustion of methane gas results in the production of carbon dioxide
and water according to the equation:
|
Eight
(8)g of methane is consumed in this reaction at an initial and final
temperature and pressure of
![]() and
3 atm, respectively:
and
3 atm, respectively:
(a)
If
initially there is exactly one mole of methane present for every two
moles of oxygen, what are the partial pressures of the methane and
oxygen gases prior to combustion?
![]()
![]()
![]()
(b)
Calculate the number of moles of methane consumed.
![]()
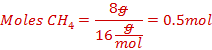
(c)
How many moles of carbon dioxide will be produced?
![]()
(d)
Calculate the volume of carbon dioxide produced.
![]()
![]()
![]()
![]()
![]()